For more questions, please click "Consult Now", a professional consultant will answer your questions.
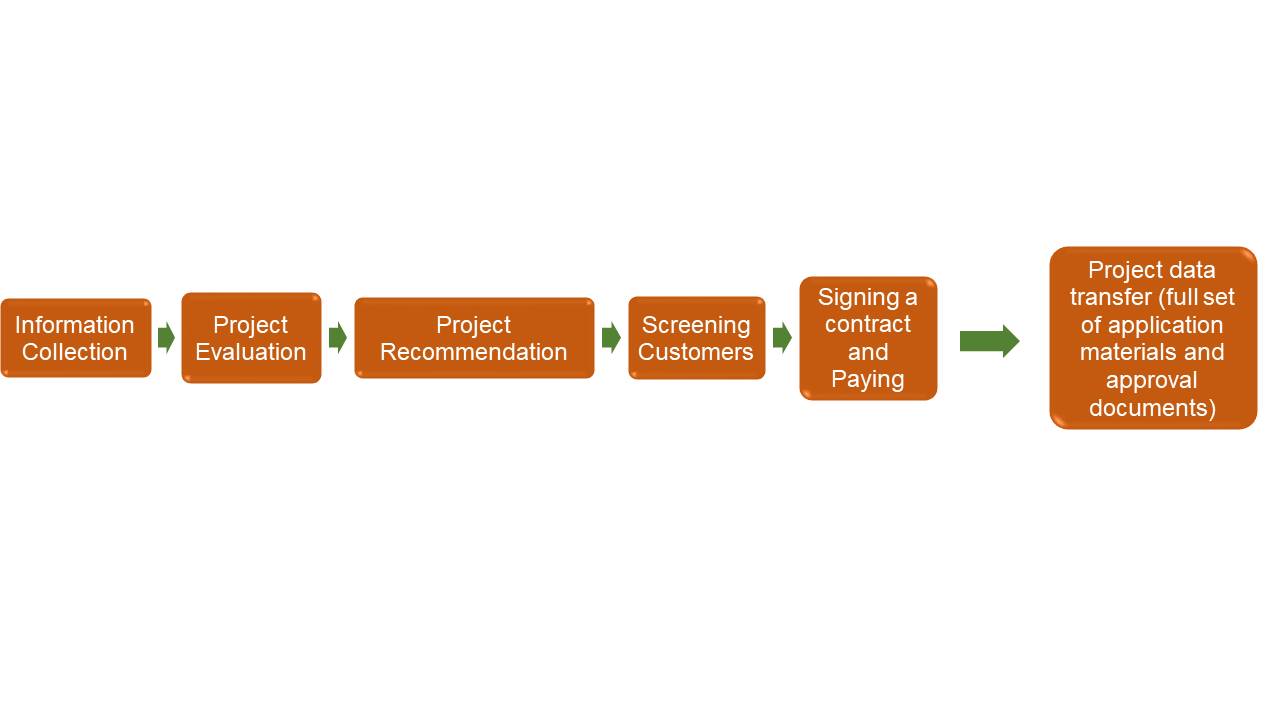
The process of technology transfer service is as follows:
(1) Information collection: collect information on new drug projects at various stages of new drug research and development, complete the writing of project contract materials and preliminary research.
(2) Project evaluation: technical and market evaluation of all new drug projects.
(3) Project recommendation: recommend the project to client, making them fully understand the project’s prospects and advantages.
(4) Screening clients: evaluate the technical level, economic strength and research and development level of clients who intend to purchase and select key clients for business negotiations.
(5) Sign technology transfer contracts with clients.
(6) Transfer project materials to clients. After the contract comes into effect, the project's drug clinical trial approval, preclinical research data, clinical research data and other technical information should be submitted to and signed by the client.