

























For more questions, please click "Consult Now", a professional consultant will answer your questions.
Boji Pharmaceutical Evaluation Center (the Center) is a technical service platform that devotes to research on the efficacy and safety of innovative drugs. Our laboratories have passed national GLP certification and obtained GLP certification. The center currently has about 6,000 square meters of animal laboratories and auxiliary facilities, including animal testing facilities of more than 3,000 square meters, functional laboratories, office areas, and auxiliary areas of about 2,000 square meters. Non-clinical evaluation of more than 10 innovative drugs can be carried out simultaneously. The center is equipped with nearly 30 million advanced instruments. It includes physiological signal telemetry system, three biopac 16-channel physiological recorders, animal color Doppler, Doppler laser blood flow meter, blood oxygen analyzer, three laser flowcy tometer, lung function detector, automatic biochemical analyzer, blood cells analyzers, two sets of pathological examination equipment, three LCMS / MS,etc. These instruments can meet the efficacy, pharmacodynamics, pharmacokinetics, and safety evaluation demands of traditional Chinese medicine, chemical medicine, biological products and some medical devices.
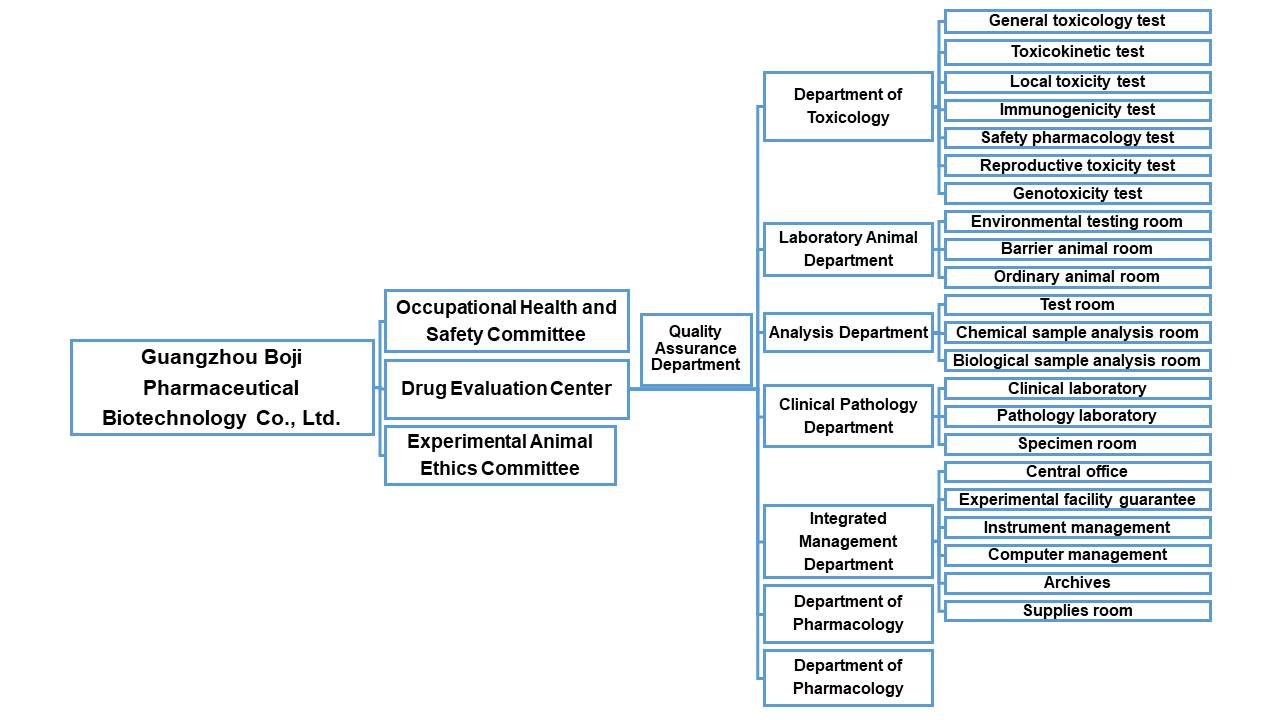
The center has 8 departments and 2 committees, including the pharmacology department, toxicology department, quality assurance department, laboratory animal department and laboratory animal ethics committee. Professional and technical personnel in pharmacology, toxicology and animal medicine are available. Senior personnel (including master's degree or above) are nearly 50%. With a perfect quality assurance system and a strong research team, the evaluation center has rich research experience in the screening and evaluation of chemical drugs, TCM and medicaldevices, non-clinical safety evaluation of drugs (including pre-tests) and pharmacokinetic studies.
Service Content:
Compound activity screening, drug ability studies (NGLP)
Research on main efficacy and mechanism of action (NGLP AND GLP)
Non-clinical safety evaluation (GLP)
Non-clinical pharmacokinetic studies (NGLP AND GLP)
Animal or human Bioequivalence Analysis (BE) and Blood Concentration Test (NGLP AND GLP)
Biochemical pathology analysis services, animal model replication, animal management and other single technical services.
|
| ||
Laboratory Animal Use Permit |
| Drug GLP certification | Certificate of Pharmacokinetic Testing Ability |
|
|
|
|
Drug Evaluation Center | Animal room | Clinical laboratory, physiological testing, surgical anatomy room | Test product analysis room |

