

























For more questions, please click "Consult Now", a professional consultant will answer your questions.








Guangzhou Jiutai Medicine & Medical Device Technology Co., Ltd. (referred to Jiutai) is a third-party service-outsourcing Contract Research Organization (CRO), which is dedicated to providing CRO services for medical devices. Jiutai is composed of a professional team, who are experienced in clinical trials, proficient in medical device policies, laws and regulations and skilled in medical device registration application. Reinforced by its parent company, namely Boji, which has been devoted to clinical trials for nearly 20 years, Jiutai has a rich practical experience in clinical trial services for Class III high-risk medical devices, especially innovative ones in the professional fields of cardiology, oncology, brain science, interventional medicine, artificial intelligence(AI), etc.
Jiutai adheres to the operation philosophy of “Preciseness, Professionalism, Harmonization, Win-win”. Jiutai has been vigorously strengthening the improvement of technical strength of its own and the construction of brand image while market development is being carried out in full swing. Jiutai endeavors to help establish the industry image of professionalism, preciseness and responsibility in the industry of medical device clinical trials and manages to contribute to the development of medical device industry.
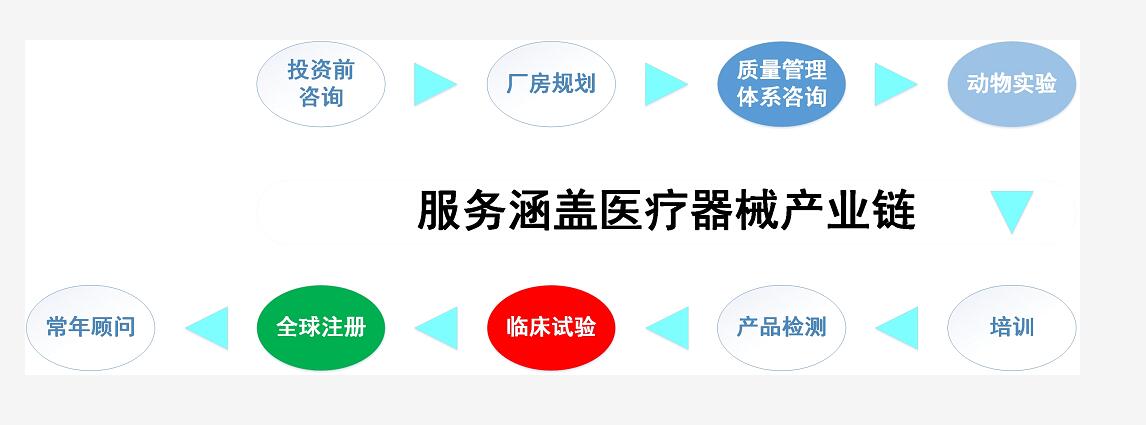
Jiutai provides third-party outsourcing services for medical devices, among which, the core technical ones are Class III medical device clinical trials, innovative medical device clinical trials, Class II medical device clinical trials, medical device post-market clinical trials, medical device real world studies, all sorts of clinical evaluations, registration application to the NMPA, pre-clinical animal experiments, quality management system establishment, training services, etc.
Major services:
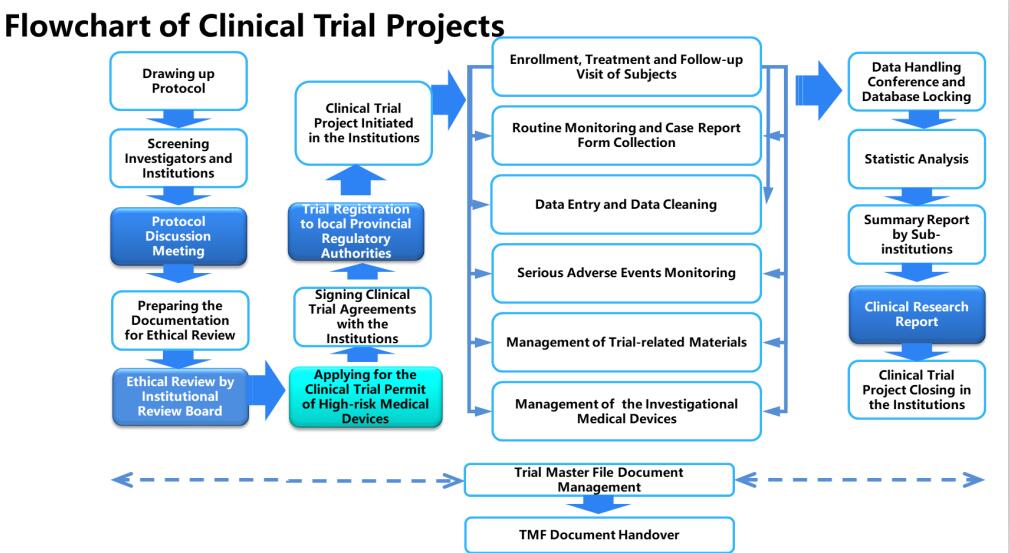
1.Clinical Study Services----Medical Device Clinical Trials
★Clinical Evaluation for Class II and Class III Medical Device
★Clinical Trials for Class II and Class III Medical Device
★Medical Device Real World Studies
★Medical Device Post - Market Clinical Studies
★In Vitro Diagnostic Reagents (IVD) Clinical Trials
★Clinical Trials for Foods for Special Medical Purpose
★Clinical Trial Protocol Design
★Data Management and Statistic Analysis
★Clinical Trial Report Writing
★Clinical Trial Monitoring and Subject Recruitment Services
2.pre-clinical services
★Investment Assessment for Significant and Innovative Medical Device Projects
★Advisory Services for Medical Device Industrial Parks
★Medical Device Animal Experiments
★Advisory Services for Medical Device Testing
3.Registration Advisory Services
★ NMPA Registration Advisory Services for Class II and Class III Medical Devices
★ NMPA Advisory Services for Medical Device Marketing Authorization Holder (MAH)
★ NMPA Registration Advisory Services for In Vitro Diagnostic Reagents (IVD)
★ NMPA Registration Advisory Services for Imported Medical Devices
★ European Union CE Certification Advisory Services
4.Professional Training
★ Training in Medical Device Clinical Trials
★ Training in Chinese Laws, Regulations on Medical Device Supervision
★ Training that Combines Medical Device and Clinical Medicine
Work Qualifications in Social organizations
Member of China Advisory Council of DIA(ACC)
Senior Consultant of US Gerson Lehrman Group (GLG)
Senior Consultant of Australia China Health Accelerator (ACHA)
Associate Editor of Prevention and Treatment of Cardiovascular Disease Magazine
Associate Editor of Blue Book of Medical Device Industry: Annual Report on the Development of Medical Device Industry in China (2019)
Associate Editor of Blue Book of Medical Device Industry: Annual Report on the Development of Medical Device Industry in China (2018)
Chief Editor of Blue book on the development of blood purification industry in China (2016)
Director General of CRO Committee of China Quality Association for Pharmaceuticals
Vice Chairman and Secretary General of Engineering-Medicine Transformation and Health Industry Convergence Committee of Chinese Research Hospital Association
Member of the Standing Committee of Medical Device Quality Management Committee of China Quality Association for Pharmaceuticals
Member of Medical Device Regulation Research Committee of China Society for Drug Regulation
Member of Clinical Trial Committee of China Association for Medical Devices Industry (CAMDI)
Vice Chairman of Clinical Trial Committee of Guangdong Medical Devices Management Academy
Guangdong Province Master Student Combination Culture Demonstration Base
Organization Member of Medical Device Panel of Guangdong Food & Drug Technology Association for Evaluation and Certification (GD•FDTAEC)
Member of Hubei Drug and Medical Device Clinical Evaluation Society
|
|
|
|
|
|  |
|
|  |  |  |
 |
|  | To be continued... |
For the protection of cooperative partners’ confidential information, we can just publicize some of medical device project experiences.
1.Carbon Ion Treatment System for the Treatment of Malignant Tumor
2.Implanted Magnetic and hydrodynamic Suspension Ventricular Auxiliary Device for the treatment of heart failure
3.Left Atrial Appendage Closure System for the prevention of thrombo embolism and risk reduction of embolism in left atrial appendage
4.Thrombectomy Device for patient with acute ischemic stroke
5.Hydrogen Generator With Nebulizer for the improvement of Acute episode COPD
6.Artificial Bone for the bone defect filling inorthopedic surgery
7.Biological Amniotic Membrane for the repair of superficial corneal defect
8.Bio-artificial Cornea for the cornealt ransplantation
9.Blood Purification Equipment for the blood purification treatment in nephrology department, emergency department, gastroenterology department and hepatology department
10.Single-use High-throughput Hemodialyzer for maintenance hemodialysis
11.Deep Brain Electrode for the monitoring of abnormal discharge at epilepsy lesions in deep brain during epilepsy surgery
12.Deep Brain Stimulator for treatment of Parkinsonism
13.Glioma Electric Field Treatment System for new orrecurrent glioblastoma
14.Magnetic Resonance Imaging (MRI) for the imaging diagnosis of various systems of the body
15.Electronic Ureter Pyeloscope for the percutaneous nephroscope set examination and treatment
16.Electronic Bronchoscope for the observation, diagnosis and photography of bronchus as a video monitor
17.Endoscopic Ultrasound System for the clinical examination at upper gastroin testinal tract
18.Optical Interference Tomography System for the examination at orifice and respiratory tract
19.Electronic Larynx Speaker for the restoration of language function as a tool to provide pronunciation power and fundamental frequency
20.Artificial Intelligence Software for the auxiliary diagnosis of diseases
21.High Potential Therapeutic Apparatus for the treatment of insomnia
22.Multimedia Visual Function Training System for amblyopia training
23.Ultrasound Scoliosis Assessment System for early discovery and monitoring teenager scoliosis
24.Blood Sugar Test Strip for adult’s and newborn babies’ blood sugar concentration
25.Patients Derived Organoids (PDO) Drug Screening IVD Kit
26.High-Throughput Drug Sensitivity Screening Technology for liver cancer
27.High-Throughput Drug Sensitivity Screening Technology for hemopathy
28.RIDA CHIP Food Specificity IgG Detection Kit
29.Hydatid IgG Antibody Detection Kit (Enzyme linked immunosorbent assays)
30.Coxsackie virus IgM Antibody Detection Kit
31.........

