

























For more questions, please click "Consult Now", a professional consultant will answer your questions.








Re-evaluation after marketing business is responsible for the professional business planning team and specialized clinical execution team of Boji Pharmaceuticals, which has rich clinical practice experience. Our rich experience makes us your best partner for re-evaluation after marketing. The characteristics and requirements of each project undertaken by our company will be fully, comprehensively and thoroughly taken into consideration. We will work with experts to carry out top-level designs and organize a professional team to ensure the realization of your project’s goal.
1、Meaning of Re-evaluate aftermarketing
Medicine re-evaluation after marketing is about making scientific evaluation and estimates of whether the approved medicines have met the principles of safe, effective and economical use of medicine in terms of efficacy, adverse reactions, medication regimen, stability and cost in social population, pharmacology, pharmacy, clinical medicine, pharmaceutical epidemiology, pharmacoeconomics and drug policy.
2、Purpose of Re-evaluate aftermarketing
Re-evaluation after marketing is an important part of medicine research and a means to ensure safe and effective use of drugs. It is also an extension of new drug evaluation. Through systematic, standardized and rigorous evaluation after marketing, the following objects[A2] are achieved:
Enterprises can comprehensively obtain effective and safe information after medicine is marketed;
Evaluate the benefits and risks of marketed medicines, provide a scientific basis for the better use of medicine;
Provide timely and scientific technical evidence for government health decision, corporate marketing decision and public health protection.
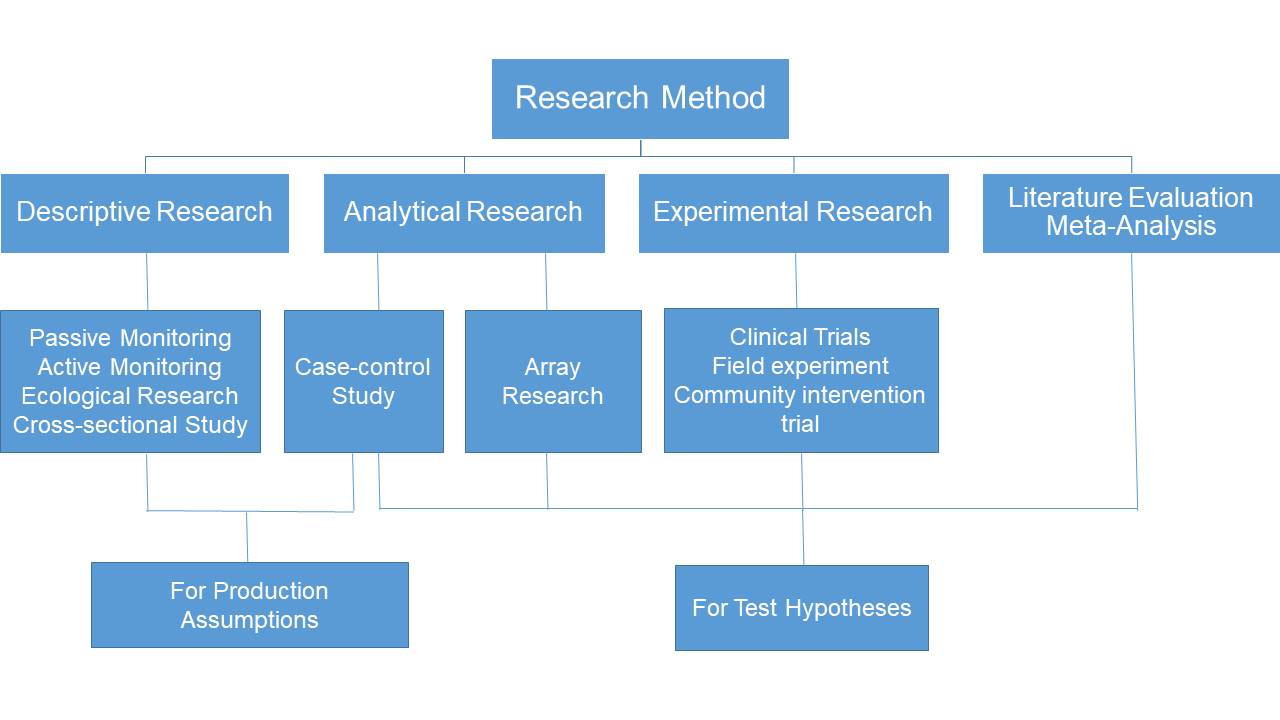
3、Type of Re-evaluate project
1) Re-register Phase IV clinical trial
2) Re-evaluation after the launch of traditional Chinese medicine injections
3) Evidence-based medicine research
4) Chinese medicine protection clinical trials
5) Evaluation of pharmacoeconomics
6) Real-world research
4、Common research method of Re-evaluate
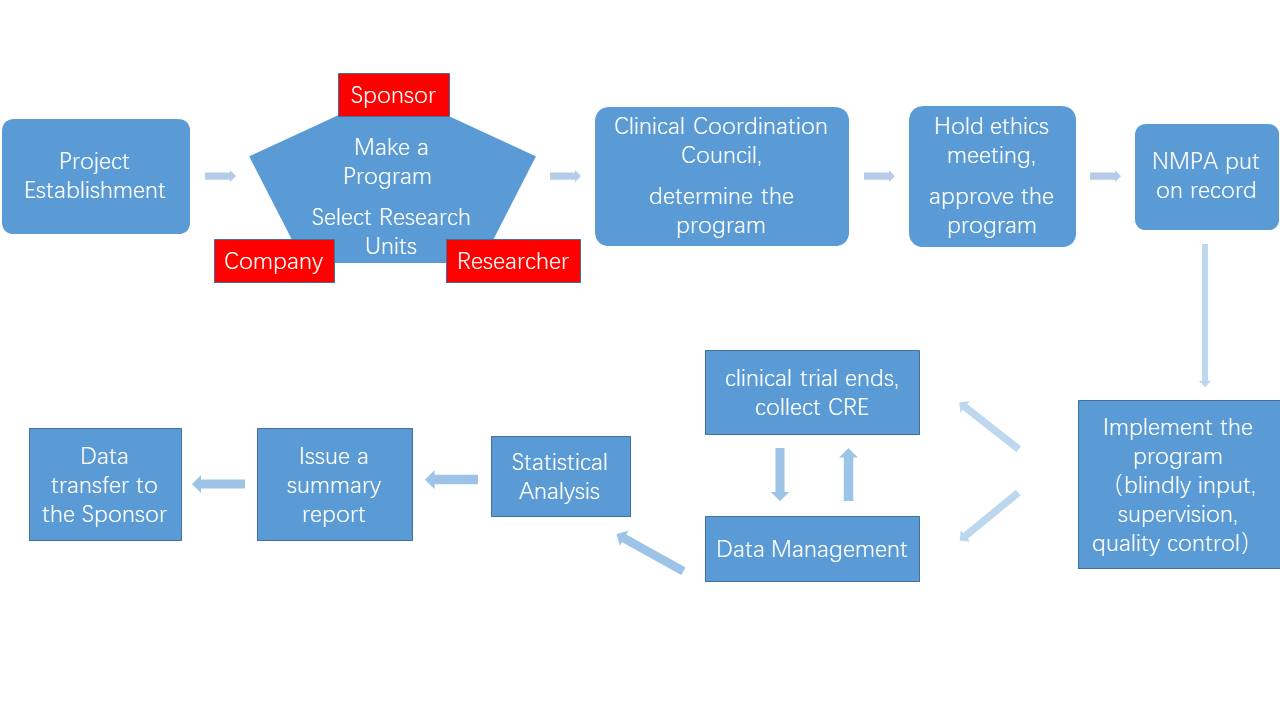
Since its establishment, the company has provided pharmaceutical research, pharmaceutical research, toxicology research and other technical services for nearly 300 customers.
1. Hydrogen and Oxygen Atomizer—a medical device for the adjuvant treatment of COVID-19 (led by Zhong Nanshan, Academician of Chinese Academy of Engineering);
2. TPN729—Category1 of Chemical Drugs, for the Treatment of Erectile Dysfunction (ED);
3. Category 1 of Chemical Drugs, for the Treatment of Hepatitis B;
4. HEmay020 Capsules—Category 1 of Chemical Drugs, for the Treatment of Non-Small Cell Lung Cancer (NSCLC);
5. YPS345 Tablets—Category 1.1 of Chemical Drugs, for the Treatment of Respiratory Diseases;
6. EPO Fusion Protein—Category 1 of Therapeutic Biological Products, for the Treatment of Renal Anemia;
7. SK08—Category 1 of Therapeutic Biological Products, for the Treatment of Irritable Bowel Syndrome (IBS);
8.Piper Phentonamine Hydrochloride—Category 1 of Chemical Drugs, for the Treatment of Acute Heart Failure;
9. Xiongdin Afilcitrate—Category 1 of Chemical Drugs, for the Treatment of Erectile Dysfunction(ED);
10. MRX-Itablets—Category 1 of Chemical Drugs, for the Treatment of Skin Infections;
11. L-phencynonate Hydrochloride Tablets—Category 1 of Chemical Drugs, for the Treatment of Motion Sickness;
12. Ginkgolide B for Injection—Category 1 of Traditional Chinese Medicines, for the Treatment of Acute Ischemic Stroke;
13. Pre-IND Application: 765IGF-MTX—An innovative drug intends to conduct international multi-center clinical trials (Hyperosteogeny Syndrome)
14. Pre-IND Application: YJ001 for spray—Category 1 of Chemical Drugs, for the Treatment of Diabetic Peripheral Neuralgia in Zhejiang;
15. Pre-IND Application: Interferon (Orphan Drug)—Category 1 of Chemical Drugs;
16. .Pre-IND Application: SI-006— Category 1 of Therapeutic Biological Products;
17. Imported Drug Registration: Masetinib mesylate tablets, for the treatment of prostate cancer, melanoma and pancreatic cancer;
18. Artificial Heart—Category Ⅲ of Medical Devices, for the Treatment of Refractory End-stage Heart Failure;
19. Left Atrial Appendage Occluder System—Category Ⅲ of Medical Devices, for the Treatment of Nonvalvular atrial fibrillation (led by Ge Junbo, Academician of the Chinese Academy of Sciences).

